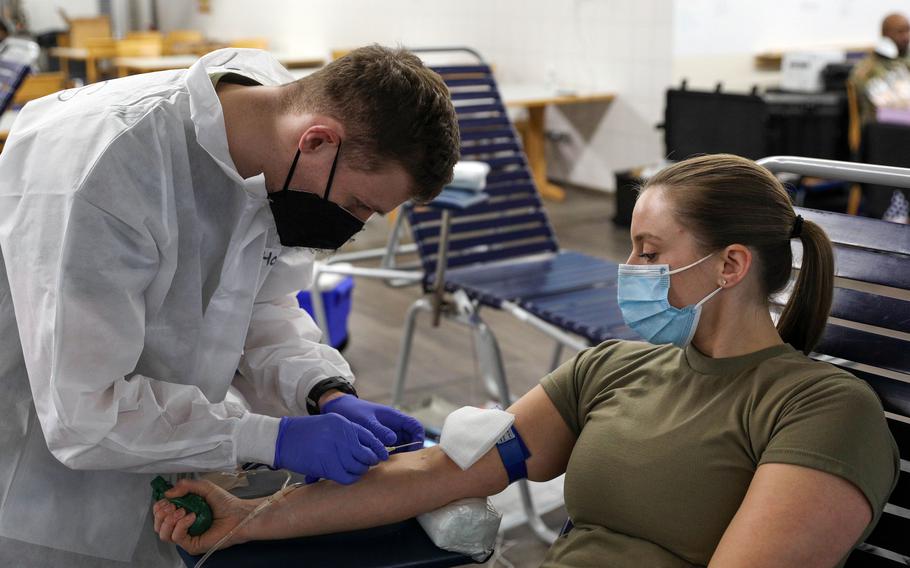
Capt. Shelby Aleksick, a nurse with the 30th Medical Brigade, donates blood in January 2022 at Rhine Ordnance Barracks in Kaiserslautern, Germany. Service members, veterans and civilians once barred from donating blood because they lived in Europe decades ago during transmission of mad cow disease may now again give blood. (Katelyn Myers/U.S. Army)
Service members, veterans and others who were once barred from blood donation because they had lived in Europe decades ago at the height of transmission of “mad cow disease” are now eligible at military donor centers.
The U.S. Food and Drug Administration recently removed restrictions, following a less inclusive change in 2020, that for decades had prevented some 4.4 million veterans, service members and Defense Department civilians from donating blood, according to the Department of Veterans Affairs.
“Donors who were previously deferred for mad cow disease risk can now give blood directly to the military at (Armed Services Blood Program) blood drives on military installations throughout Europe,” program officials said in a statement Wednesday.
“They can help save the lives of U.S. and NATO military on and off the battlefield as well as patients in U.S. military hospitals around the world like Landstuhl Regional Medical Center” in Germany, the statement said.
The American Red Cross said it also will change and simplify its recommendations to defer donations from people who spent time in certain European countries or on military bases and were previously considered to have been exposed to a potential risk of transmission of the disease.

The Armed Services Blood Program-Europe visited RAF Lakenheath, England, in 2018 to host a mobile blood drive. Service members, Defense Department civilians and veterans once barred from donating blood because they were stationed in Europe decades ago during transmission of mad cow disease may now again give blood. (Shanice Williams-Jones/U.S. Air Force)
Mad cow disease, also known as variant Creutzfeldt–Jakob disease, is attributed to human infection with the agent of bovine spongiform encephalopathy, likely from consumption of contaminated beef products, according to the FDA.
The rare but invariably fatal disease was first recognized in the U.K. in 1985 and subsequently spread worldwide.
It also can be transmitted through blood transfusions from an infected donor, and there is no blood test for it. The FDA issued its first guidance restricting blood donors in 1999.
In 2020, the FDA lifted restrictions affecting people who had spent time or received a blood transfusion in European countries except the U.K., Ireland and France.
But it continued to recommend indefinite prohibition of blood donations by those who had spent time in the U.K. from 1980 to 1996 or in either France or Ireland from 1980 to 2001, as well as those who received a blood transfusion in any of those three countries from 1980 to the present.
In a May announcement, the FDA recommended that those geographically based restrictions be canceled, too. Risk assessments by the agency after years of research determined that allowing such potential donors would pose little or no risk to the blood supply.
As of April 2021, there have been 232 cases of the disease worldwide, 178 of which were in the U.K., 28 in France, four in Ireland, four in the U.S. and 18 in eight other countries, according to the FDA.
According to the FDA, the COVID-19 pandemic has caused “unprecedented challenges” to the U.S. blood supply because of social distancing requirements and the cancellation of blood drives.
The ASBP’s lone European blood donation center is in Landstuhl, Germany. The team at the center conducts weekly blood drives at local military installations, with annual blood drives at other locations throughout Europe.